Drug Delivery
It is a truism that pharmaceutical research is not possible without patent protection. The 20 or 21-year term of a patent is more than sufficient for many branches of industry, but not so in the field of pharmaceuticals, because the lengthy clinical test phases and approval procedures mean that it can sometimes take 15 years before the first commercial exploitation is possible. The remaining time is then no longer sufficient to recover the development costs, let alone make a profit.
The legislator has recognized this and, with the so-called „Supplemental Protection Certificates“, offers a possibility to extend the patent protection for active ingredients from the fields of pharmaceuticals and crop protection by 5 years, provided that an approval exists for the substances. Depending on when this occurs, however, even an SPC may only provide a small additional period of patent protection.

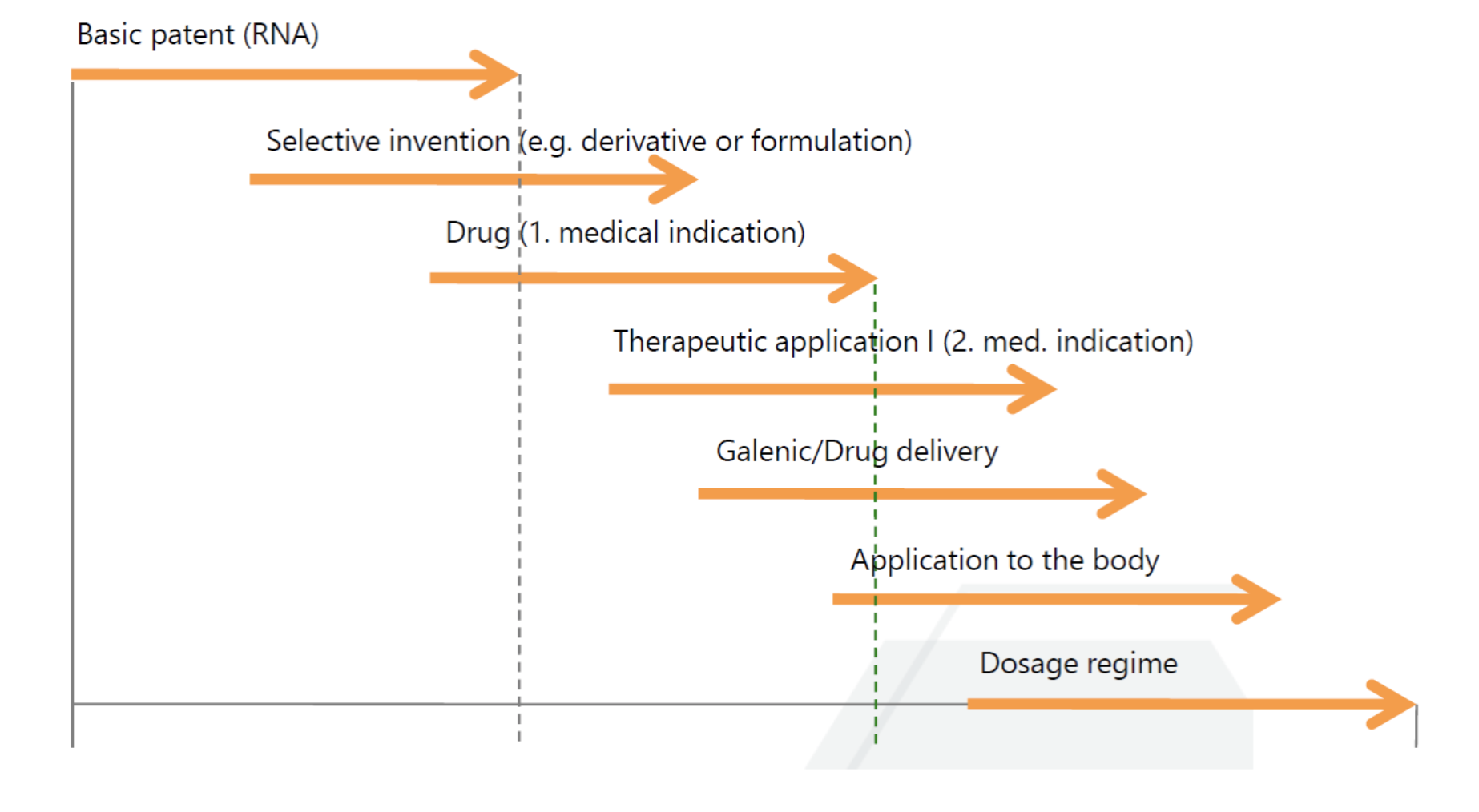
Life cycle management in the pharmaceutical industry
It is therefore the aim of any patent-strategic life cycle management to extend the period of protection for an active ingredient by staggering selection inventions over the term of the basic patent, as exemplified in the figure on the left side.
Drug Delivery
In addition to applications relating to further pharmacological effects („2nd medical indication“), solubility enhancement by claiming special salts and solvates, forms of administration (oral, subcutaneous, etc.) and dosage regimes, the galenic of the active ingredients, so-called „drug delivery“, is one of the most important strategic claim topics. In fact, in most cases, the form of delivery proves to be as important as the active ingredient itself. The way in which an active ingredient is prepared for application depends on the purpose to be achieved. Basically, three requirements can be distinguished:
1. How can the active ingredient be transported to the specific location in the organism (e.g., a cell) so that it can exert its effect there?
2. How can the active ingredient be protected during oral uptake so that it is not destroyed on its way through the body, for example by the acidic pH in the gastrointestinal tract?
3. How can the bioavailability of the active ingredient be extended over a longer period of time, for example by controlled release?
Macro- and microcapsules
Encapsulation is suitable for protecting an active ingredient against premature metabolization or release. In this process, the active ingredient is encapsulated in a shell that has to meet the respective requirements, e.g., be resistant to high or low pH values for a certain period of time or degrade only slowly in a certain environment, thus releasing the active ingredient gradually. A distinction is made between macro-capsules with diameters of about 0.1 to about 5 mm or micro-capsules with diameters of about 0.0001 to about 0.1 mm. The capsules are generally finely dispersed liquid or solid phases coated with film-forming polymers, during the production of which the polymers are deposited on the material to be coated after emulsification and coacervation or interfacial polymerization.
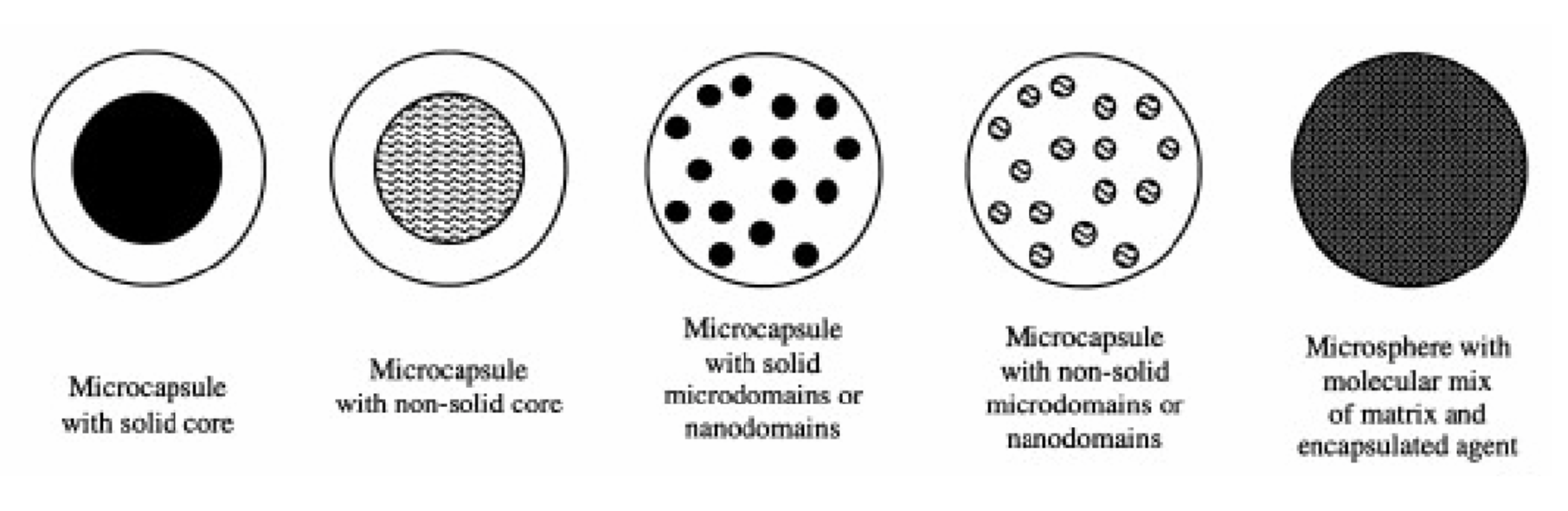
For industrial purposes, for example, urea-formaldehyde capsules can be considered, but these may not be used in cosmetics or pharmaceuticals. There, mainly coacervates of anionic and cationic polymers are used. From the prior art, there are thousands of patents and patent applications in various technical fields dealing with encapsulation technologies and it is amazing that there is still room for improvement inventions.
Vectors and nanoparticles
However, in order to introduce active substances into cells in a targeted manner, the known encapsulation technologies are ruled out. Instead, biomolecules, such as DNA, RNA, or peptides, are reversibly delivered through the cell wall using carriers known as vectors, which are then released.
To produce these vectors, the carrier – usually a polymer – is introduced into the oil phase of a microemulsion. By using double emulsion techniques (water/oil/water), hydrophilic molecules, such as most biomolecules, can be incorporated into the polymer matrix or docked onto the polymer surface, obtaining nanoparticles. When the solvent is removed by evaporation or extraction, the vector consisting of carrier and biomolecule remains. Peptides entrapped in such nanoparticles are protected from enzymatic degradation and are released only slowly. This property has been used as an advantage for injectable depots, but can be detrimental to bioavailability when administered orally.
The first generation of these vectors included cationic polymers such as poly(ethyleneimine) (PEI) and poly(lysine), but their use was associated with a significant degree of cytotoxicity. Therefore, high gene expression was usually achieved only at the expense of cell viability. In addition, PEI is not biodegradable and may not be safe for long-term treatment of patients.
Instead, branched poly-beta-amino esters (PBAE), which have a cationic character in physiological solutions and can therefore bind nucleic acids, represent the state-of-the-art. They are both, biodegradable and toxicologically safe. They are also suitable for transporting active substances directly into the epithelial cells of the lung by nebulization, for example. These vectors were first patented by the Agency for Science Technology and Research (AStar). Important patents in this field are held by MIT and John Hopkins University. Nevertheless, there are a number of ways to avoid costly licensing here by switching to PBAEs, which are free prior art.
Interested?
If you have any questions or suggestions do not hesitate to contact us.
