Controlling COVID19 infections is essential to prevent further damage to society and the collapse of health care systems.
PCR-based methods have been the gold standard testing approach for the first phase of this pandemic. However, low throughput, high costs and long processing times are limiting the efficiency of identifying infectious people. Recently established alternatives for SARS-CoV2 detection such as RT-LAMP can improve our current testing strategies and make large scale testing of society a reality. Additionally, reduced costs and accessible open-source reagents used in RT-LAMP have the potential to bring effective tools to suppress infection rates even to the most rural areas of the world.
qRT-PCR: Our most important weapon against COVID19 until now
The emergence of the novel SARS-CoV2 virus has turned our lives upside down. While we begin settling into a new “normal”, the recent spike of infections in Europe and all over the world are a stark reminder that this pandemic is far from over. However, advances in biomedical research over the last century equip us with tools that can help to reduce the duration of the pandemic or stop it in its tracks entirely. Several promising vaccine candidates are currently in phase III clinical trials and the probability of an efficient protection against COVID19 in the near future is high. However, once a safe and functional vaccine is found, the logistics involved in distributing this tool to all corners of the globe are highly complicated and large-scale immunisation of populations will require month if not years illustrating the need for the implementation of strategies that control infection numbers.
In the short term, therefore, our aim has to be the reduction of infection numbers by identifying virus carriers and isolating them from society for an appropriate time. Initially, the sole and most reliable method to test for infections was through detection of viral RNA from throat or nasal swaps by quantitative reverse transcription polymerase chain reaction (qRT-PCR), a standard molecular laboratory technique. However, high costs, expansive equipment, the low throughput, and the need for skilled personal make this detecting method inefficient on large scales. As governments around the globe desperately try to identify alternatives for testing patients or asymptomatic carriers, the biomedical community has stepped up and developed multiple alternatives that could make COVID19 testing more cost-effective, faster, and accessible than our current gold standard qRT-PCR.
Novel testing approach could be a ‘game changer’
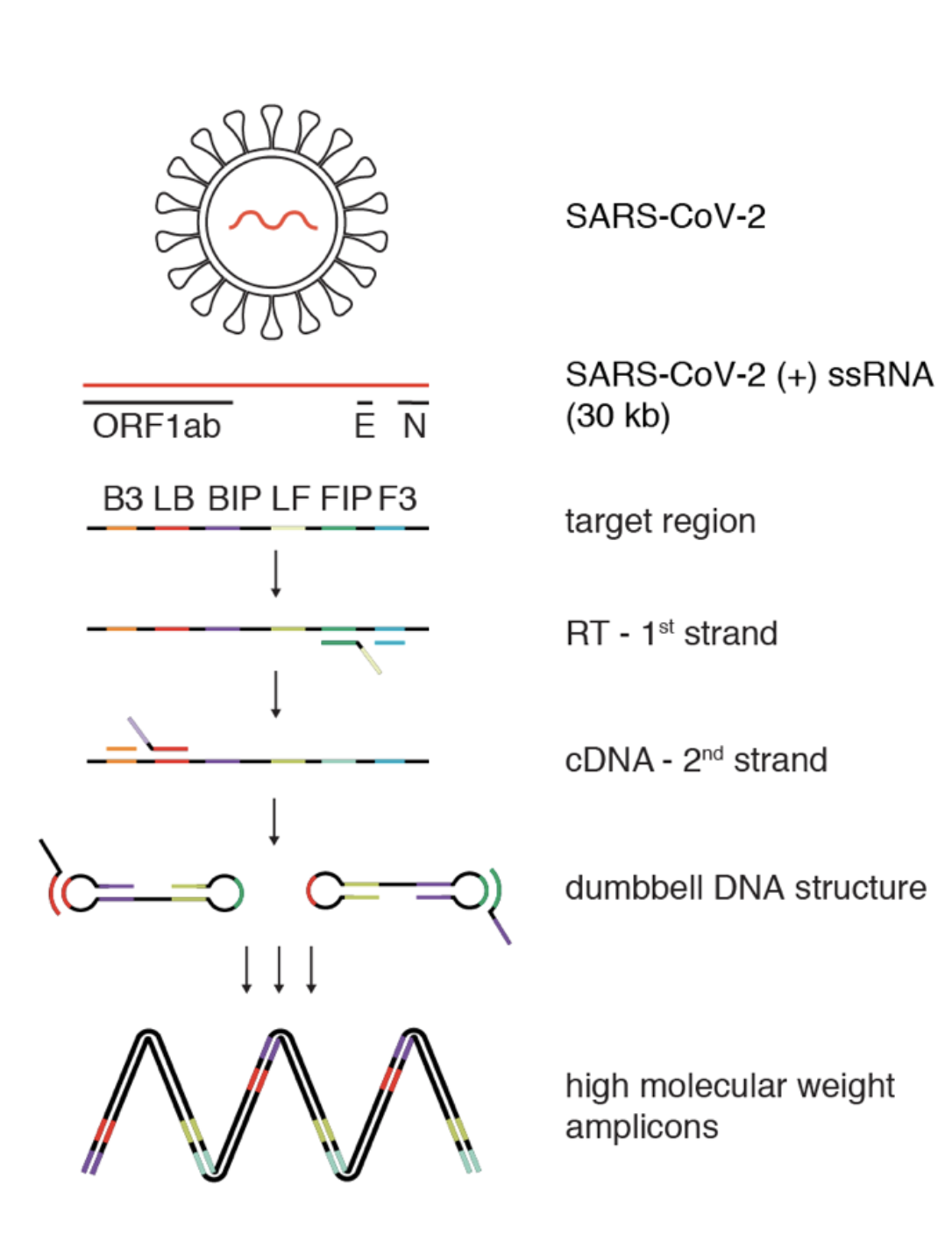
One example is the Loop-mediated isothermal amplification after reverse transcription (RT-LAMP) method, which relies on technology similar to qRT-PCR approaches but does not require high cost equipment such as thermal cyclers. Viral RNA extracted from patients is converted into single stranded DNA by an engineered reverse transcriptase and massively amplified by DNA polymerases. In contrast to qRT-PCR, this method can be performed at a constant temperature, making it much more attractive for less specialised laboratories lacking appropriate facilities. Additionally, determining positive results relies on a colour change observable by eye rather than precise measurements of qRT-PCR machines (Figure 1).
This simple but highly sophisticate method has several advantages: it allows for high throughput testing with minimal lab equipment and is much cheaper compared to PCR-based methods (< 2 USD/sample for commercially available RNA extraction and RT-LAMP reagents). Additionally, the short reaction time of approximately 30min allows greatly reduced turnaround times for a high number of samples. qRT-PCR -based detection methods require the purification of viral RNA, which is costly, requires again special equipment, and is time consuming. Recent advances of RT-LAMP enable the use of patient samples without RNA purification as input material, further reducing cost and testing time. The simple workflow of RT-LAMP in comparison to qRT-PCR could enable large-scale testing in care homes, schools, and at airports without the need for expansive reagents and equipment making COVID-19 testing accessible for everyone at any time.
Figure 1: A sensitive, robust RT-LAMP assay compatible with crude patient samples.
Schematic illustrating loop-mediated amplification (LAMP) of SARS-CoV-2 RNA. Each target region is recognized by a defined set of primers. The RNA template (red) is reverse transcribed and displaced after first-strand synthesis; the outer primer binding sites are added in the subsequent amplification step. The resulting dumbbell DNA structure acts as template for further rounds of amplification, ultimately leading to high molecular weight amplicons. Modified from Kellner et al., 2020. Recent spikes of infections in developing countries are a great concern as medical facilities are often inadequately equipped to sufficiently treat COVID19 patients with severe symptoms such as respiratory failure. Controlling viral spread is therefore critical to prevent the collapse of health care systems. While RT-LAMP can be a cost-effective alternative for standard COVID19 testing methods with minimal equipment necessary, it still requires established supply chains for the transport of required reagents purchased from companies owning the intellectual property. Enzymes are sensitive to temperature changes and require constant access to refrigeration, known as a cold chain, illustrating the obstacles faced by rural communities to implement effective testing regimes. Manufacturing of the required reagents in local laboratories can significantly reduce the need for cooled transport and solve some of these problems. However, patent protection of the commercially used enzymes for RT-LAMP prohibits local production.
Open-Source Enzymes: Bringing testing to all corners of the world
The scientific community has worked together to allow access to reagents critical for testing without risking patent infringement. Projects such as the Open Enzymes Collection provide researcher with open-source DNA sequences encoding enzymes typically used in biotechnology under an Open Materials Transfer Agreement (OpenMTA). Production of these enzymes is simple and can be scaled up to produce large amounts of reagents used for testing without the need of supply chains, cooled transport, or purchasing of expensive licenses. A recent study combined usage of open-source wild-type reverse transcriptase and DNA polymerase with low-tech sample processing from gargle or saliva samples (Figure 2). Promising results indicate high sensitivity and fast turnaround times without the need for expansive reagents or equipment and simple sample collection. The application of these optimised RT-LAMP protocols thereby could enable local testing independently of commercially available resources (Kellner et al., 2020).
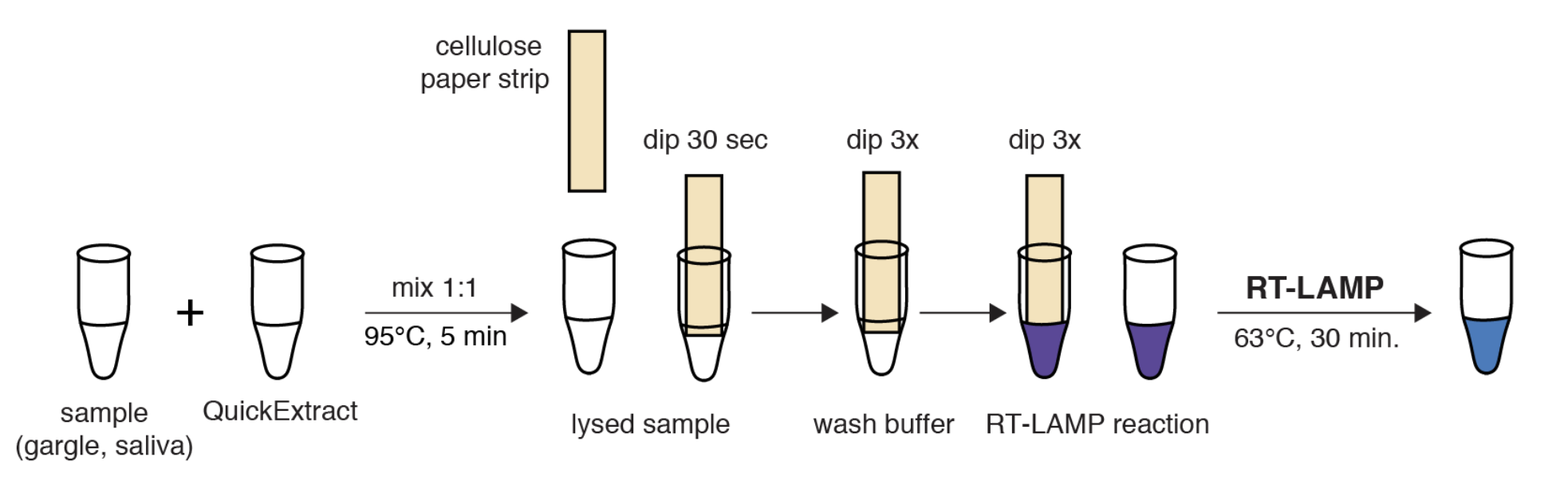
Figure 2: HomeDip-LAMP enables SARS-CoV-2 detection in low-resource and home settings
Schematic depicting the HomeDip-LAMP workflow. Samples are mixed 1:1 with QuickExtract lysis buffer and inactivated at 95°C for 5 minutes. Cellulose paper dipsticks are loaded by dipping into the crude sample for 30 seconds. After a brief washing step (3x dipping into wash buffer), RNA is released into pre-distributed RT-LAMP reaction mixes by 3x dipping. RT-LAMP reactions are performed in a water bath at 63°C and read out after 35 minutes. Modified from Kellner et al., 2020
Affordable and simple testing approaches are vital to decrease viral spread. As we approach winter and our social life more and more concentrates indoors, accessible and largescale testing is key to prevent further COVID19 outbreaks and safe lives. RT-LAMP is a promising tool to bring testing to less specialised laboratories around the world and can help to keep society open. While developed nations have the financial means and purchasing power to make routine screening a reality, bringing testing to rural and poorer areas is a constant challenge. However, the recent advances of RT-LAMP approaches implementing locally sources reagents and equipment can further improve distribution and access in developing countries. This strategy will provide communities with simple, cost-effective testing thereby protecting societies and fragile economies form further damage inflicted by COVID19.
References and resources:
Kellner, M.J., Ross, J.J., Schnabl, J., Dekens, M.P.S., Heinen, R., Grishkovskaya, I., Bauer, B., Stadlmann, J., Menéndez-Arias, L., Fritsche-Polanz, R., et al. (2020). A rapid, highly sensitive and open-access SARS-CoV-2 detection assay for laboratory and home testing. BioRxiv 2020.06.23.166397.
https://openbioeconomy.org/projects/open-enzyme-collections/

